-
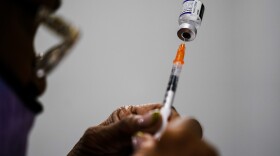
The shortage comes as there has been a steady increase in rates of syphilis since 2000. Inventory can start depleting as early as this month, Pfizer said.
-
Pfizer said that tweaking its vaccine to better target the omicron variant is safe and works — just days before regulators debate whether to offer Americans updated booster shots this fall.
-
Pfizer plans to submit new data to the Food and Drug Administration this week, bringing families with young children one step closer to a long-awaited vaccine.
-
Data show that a third dose can help boost kids' immunity. Some experts are skeptical that another shot is needed for younger kids.
-
NPR has obtained the government's $5.3 billion contract for the first 10 million courses of Paxlovid, an antiviral pill for COVID-19. Here's what's in it.
-
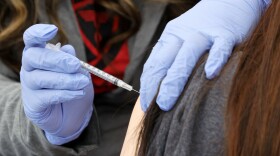
Though people who are vaccinated and boosted appear to be better protected against omicron, the highly contagious variant has still led to breakthrough cases and a surge in infections worldwide.
-
The new recommendation for adolescents age 12-17 came hours after a panel of CDC advisers voted in favor of it. The boosters should be given five months after initial immunization.
-
The move to shorten the Pfizer booster interval comes as the U.S. shatters daily case records. The recommended interval for those who received Moderna or Johnson & Johnson vaccines has not changed.
-
The authorization comes in the midst of an explosion of COVID-19 cases nationwide driven by the omicron variant — a surge that has brought a spike in pediatric hospitalizations.
-
Pfizer and BioNTech, which produced the first COVID-19 vaccine authorized in the U.S., say they will expand ongoing trials to include a third dose for children as young as 6 months old.
Play Live Radio
Next Up:
0:00
0:00
Available On Air Stations